Amino acids are organic molecules that serve as the building blocks of proteins. Each amino acid consists of a central α-carbon atom bonded to a basic amino group (−NH2), an acidic carboxyl group (−COOH), a hydrogen atom, and a unique organic R group (side chain). Proteins, which are essential for catalyzing cellular chemical reactions, providing structural elements, and binding cells into tissues, are composed of these amino acids.
Amino acids introduction
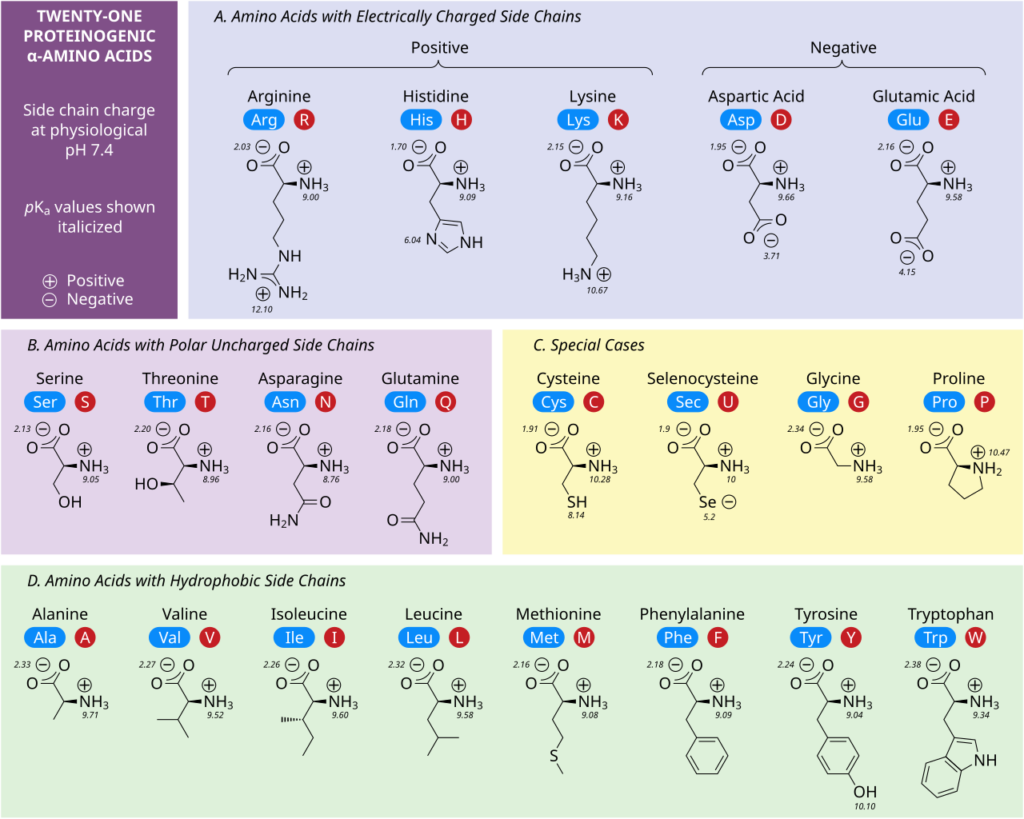
In humans, there are 20 standard amino acids. Nine of these are essential and must be obtained through the diet: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. Five amino acids are nonessential, as the body can synthesize them: alanine, asparagine, aspartic acid, glutamic acid, and serine. The remaining six are conditional amino acids, essential only during certain life stages or under specific conditions: arginine, cysteine, glutamine, glycine, proline, and tyrosine. Also, selenocysteine, sometimes considered the 21st amino acid, is derived from serine during protein biosynthesis.1
Amino acids are the building blocks of protein synthesis. They are structural elements and energy sources of cells necessary for normal cell growth, differentiation, and function. Amino acid metabolism disorders have been linked with several pathological conditions, including metabolic diseases, cardiovascular diseases, immune diseases, and cancer.
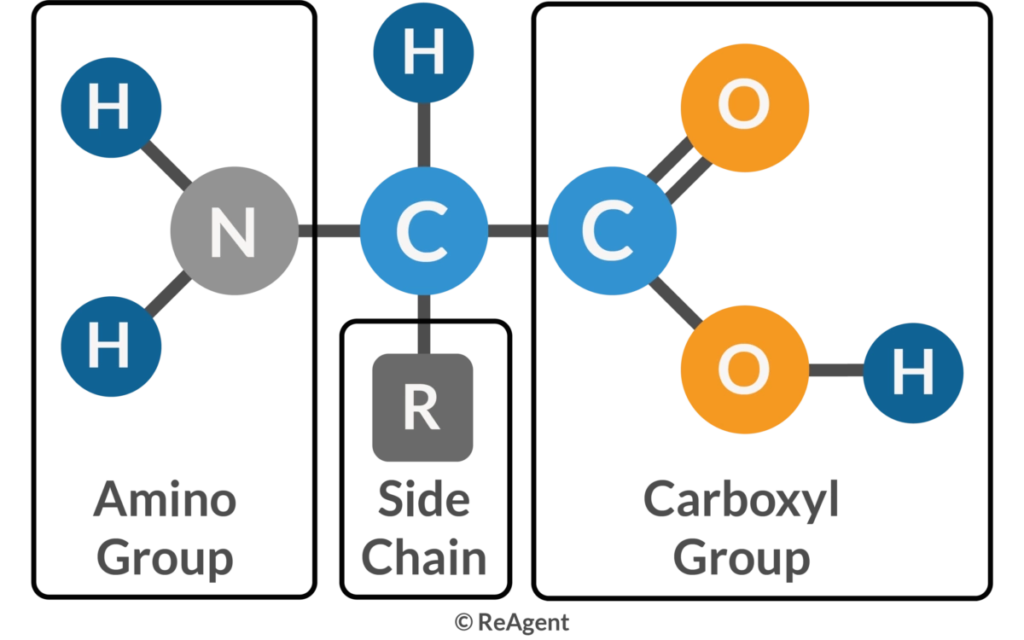
By definition, amino acids are always made up of an amino group, an acid, and a side chain. The amino group is most often adjacent to the primary carboxyl group (alpha-position) but may be in the beta position instead (e.g., in beta-alanine). As in carnitine, a tertiary amine may serve the same function. Similarly, a sulfoxide function may represent the acid group, as in taurine. The side chain may be just single hydrogen (in glycine), straight (alanine), or branched (valine, leucine, and isoleucine) aliphatic chains, contain aromatic rings (phenylalanine, tyrosine, and tryptophan), sulfur (methionine, cysteine, and taurine), selenium (selenocysteine), hydroxyl groups (serine, threonine, hydroxyproline, and hydroxylysine), second carboxyl groups (glutamate and aspartate) or third carboxyl groups (gamma-carboxyl glutamate and gamma carboxyl aspartate), amido groups (glutamine and asparagine), or additional nitrogen-containing groups (lysine, arginine, and histidine).
Names, formulas, and sidechains of the amino acids
| Name [Abbreviation (One-letter code)] | Formula | Sidechain |
|---|---|---|
| Alanine [Ala (A)] | C₃H₇NO₂ | -CH₃ |
| Glycine [Gly (G)] | C₂H₅NO₂ | -H |
| Leucine [Leu (L)] | C₆H₁₃NO₂ | -CH₂CH(CH₃)₂ |
| Valine [Val (V)] | C₅H₁₁NO₂ | -CH(CH₃)₂ |
| Threonine [Thr (T)] | C₄H₉NO₃ | -CH(OH)CH₃ |
| Serine [Ser (S)] | C₃H₇NO₃ | -CH₂OH |
| Methionine [Met (M)] | C₅H₁₁NO₂S | -CH₂CH₂SCH₃ |
| Cysteine [Cys (C)] | C₃H₇NO₂S | -CH₂SH |
| Lysine [Lys (K)] | C₆H₁₄N₂O₂ | -CH₂CH₂CH₂CH₂NH₂ |
| Arginine [Arg (R)] | C₆H₁₄N₄O₂ | -CH₂CH₂CH₂NHC(=NH)NH₂ |
| Aspartic acid [Asp (D)] | C₄H₇NO₄ | -CH₂COOH |
| Glutamic acid [Glu (E)] | C₅H₉NO₄ | -CH₂CH₂COOH |
| Asparagine [Asn (N)] | C₄H₈N₂O₃ | -CH₂CONH₂ |
| Glutamine [Gln (Q)] | C₅H₁₀N₂O₃ | -CH₂CH₂CONH₂ |
| Tryptophan [Trp (W)] | C₁₁H₁₂N₂O₂ | -CH₂C₈H₆N |
| Histidine [His (H)] | C₆H₉N₃O₂ | -CH₂C₃H₃N₂ |
| Phenylalanine [Phe (F)] | C₉H₁₁NO₂ | -CH₂C₆H₅ |
| Tyrosine [Tyr (Y)] | C₉H₁₁NO₃ | -CH₂C₆H₄OH |
| Isoleucine [Ile (I)] | C₆H₁₃NO₂ | -CH(CH₃)CH₂CH₃ |
| Proline [Pro (P)] | C₅H₉NO₂ | -(CH₂)₃NHCH₂- |
The 21 proteinogenic α-amino acids found in eukaryotes, grouped according to their side chains’ pKa values and charges carried at physiological pH (7.4)
Amino acid classifications
| Classification | Amino Acids |
|---|---|
| Simple | Glycine, Alanine, Leucine, Valine |
| Hydroxy | Threonine, Serine |
| Sulphur-containing | Methionine, Cysteine |
| Basic | Lysine, Arginine |
| Acidic | Aspartic acid (Aspartate), Glutamic acid (Glutamate) |
| Acid amide | Asparagine, Glutamine |
| Heterocyclic | Tryptophan, Histidine |
| Aromatic | Phenylalanine, Tyrosine |
| Essential | Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan, Valine |
| Semi-essential | Arginine, Cysteine, Glutamine, Glycine, Proline, Tyrosine |
| Non-essential | Alanine, Asparagine, Aspartic acid (Aspartate), Cysteine, Glutamic acid (Glutamate), Serine |
Since the alpha-carbon is chiral for all amino acids except glycine, two stereoisomeric conformations are possible. When the carboxylic group in structural diagrams is depicted at the top and the side chain at the bottom, the l form is the one that has the amino group pointing to the left, and the d form has it pointing to the right. Human proteins contain only l-amino amino acids (and glycine). d-Amino acids are quantitatively less common in mammals than in fungi and bacteria, but a few (including d-aspartate and d-serine) are synthesized by humans (D’Aniello et al., 1993; Wolosker et al., 1999, 2000).
Some amino acids form only a few specific peptides, such as taurine as part of glutaurine, and beta-alanine as part of carnosine and anserine. Several other amino acids are not a regular part of human proteins but serve specific important functions. This is the case with carnitine (fatty acid transport), taurine (osmolyte, neuronal agent, and antioxidant), ornithine (urea cycle and polyamine synthesis), and citrulline (urea cycle)
References
- Amino Acids Structure, Physical and Chemical Properties, Function, Metabolism, and Absorption: AminoADB
- Kohlmeier, M. (2015). Nutrient metabolism: Structures, functions, and genes (2nd Edition). Academic Press.
- D’Aniello et al., 1993; Wolosker et al., 1999, 2000
- Ling, ZN., Jiang, YF., Ru, JN. et al. Amino acid metabolism in health and disease. Sig Transduct Target Ther 8, 345 (2023). doi: 10.1038/s41392-023-01569-3 ↩︎
